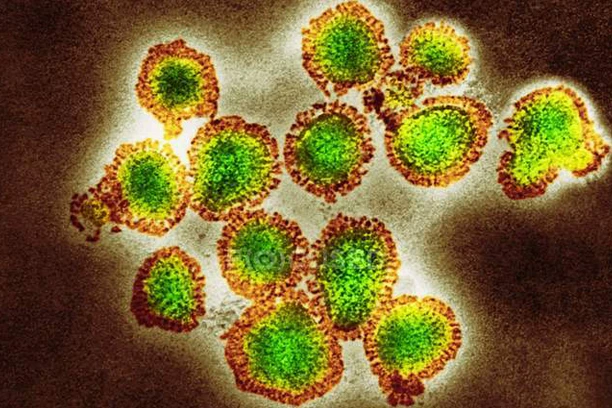
Orthomyxoviridae (Influenza)
The family Orthomyxoviridae, which includes enclosed viruses with segmented negative-sense, single-strand RNA genomes, includes influenza viruses. Types A and B are the most clinically significant of the four genera in this family (A, B, C, and D) because they cause influenza pneumonia with potentially serious side effects like encephalitis, myocarditis, and mortality. Both contaminated surfaces and airborne infections can spread the virus. It enters the body through the lower airways, including the nasal and laryngeal mucosa, and then spreads throughout the body to infect different cell types. Here’s all you need to know about Orthomyxoviridae.
About Orthomyxoviridae
Although influenza A viruses are the primary cause of pandemics and the majority of influenza cases, influenza B viruses can also be the cause of morbidity during non-pandemic seasons and up to 23% of influenza cases in a given season. It is divided into the B/Victoria and B/Yamagata genealogical lineages, which are antigenically distinct. Unlike influenza A, which can affect humans, birds, and pigs among other species, influenza B is normally only seen in humans. Antiviral drugs such as oseltamivir, marboxil, peramivir, and zanamivir are used to treat influenza. The H1N1 and H3N2 influenza A virus strains, as well as one or two influenza B virus subtypes, are frequently protected against by the influenza vaccine.
Both influenza A (IAV) and B (IBV) viruses have eight viral RNA gene segments that each encode ten essential and auxiliary viral proteins. The viral lipid membrane glycoproteins HA (hemagglutinin) and NA (neuraminidase), which identify the subtype of influenza virus, are found in IAV and IBV. Both the HA and NA proteins are targets for immune responses against the virus, and antiviral medications like Relenza and Tamiflu are directed at the NA protein. The M2 protein, which is the target of the antiviral medications amantadine and rimantadine, is also entrenched in the lipid membrane. The viral RNA segments are located inside the viral capsid, which is made up of the matrix protein M1, polymerase B1 and B2 proteins, PA (polymerase acidic protein), and NP (nucleoprotein). Additionally, the virion contains the non-structural proteins NS1 and NEP (nuclear export protein NS2).
Influenza Life Cycle
Hemagglutinin protein (HA) is used by IAVs to bind to cell surface glycoconjugates that contain terminal sialic acid residues, which causes endocytosis to occur. The resulting endosome merges with the viral membrane, releasing viral ribonucleoproteins into the cytosol. The cytosol then draws importins to aid in the viral ribonucleoproteins’ transportation into the cell nucleus through the nuclear pore complex. The viral RNA-dependent RNA polymerase subsequently copies and copies the viral RNA inside the nucleus.
In order to assemble into a viral ribonucleoprotein, the freshly duplicated RNA joins forces with nucleoprotein molecules and a single copy of the viral polymerase. The viral ribonucleoprotein is then transported to the cell surface and budded from the plasma membrane. Numerous host proteins that are engaged in various viral life cycles processes, such as viral entrance, uncoating, viral RNP nuclear import, transcription and translation, post-translational modification, and transport out of the cell, participate in the complex but quick (6-hour) replication cycle. In order to release virions and end the infectious cycle, the viral neuraminidase (NA) cleaves terminal sialic acids.
The M2 protein, which is the target of the antiviral medications amantadine and rimantadine, is also entrenched in the lipid membrane. The viral RNA segments are located inside the viral capsid, which is made up of the matrix protein M1, polymerase B1 and B2 proteins, PA (polymerase acidic protein), and NP (nucleoprotein). Additionally, the virion contains the non-structural proteins NS1 and NEP (nuclear export protein NS2).
Influenza A virions have two highly antigenic surface glycoproteins (HA and NA) that perform important functions in the viral life cycle. Host antibodies have the ability to neutralise both of these proteins, and inactivated IAV vaccinations cause antibody responses to HA and NA. However, because of the high mutational tolerance of these surface glycoproteins and the segmented nature of the genome, which makes genomic reassortment during coinfection with various strains possible, there is a chance for both antigenic drift and antigenic shift that enables escape from host immune responses.